40+ using thermodynamic data to calculate k
Show transcribed image text. To calculate ksp from ΔG of reaction.
Solved Using Standard Thermodynamic Data From Appendix D Chegg Com
2df6e43950e74971947241dc5cfa834e Standard Thermodynamic Properties for.
. Web Ksp for the reaction BaSO4s Ba2aq SO42aq is 11 1010. The relation between Ksp and ΔG is given as. Web Use tabulated thermodynamic data to calculate the standard entropy change of each of the reactions listed below.
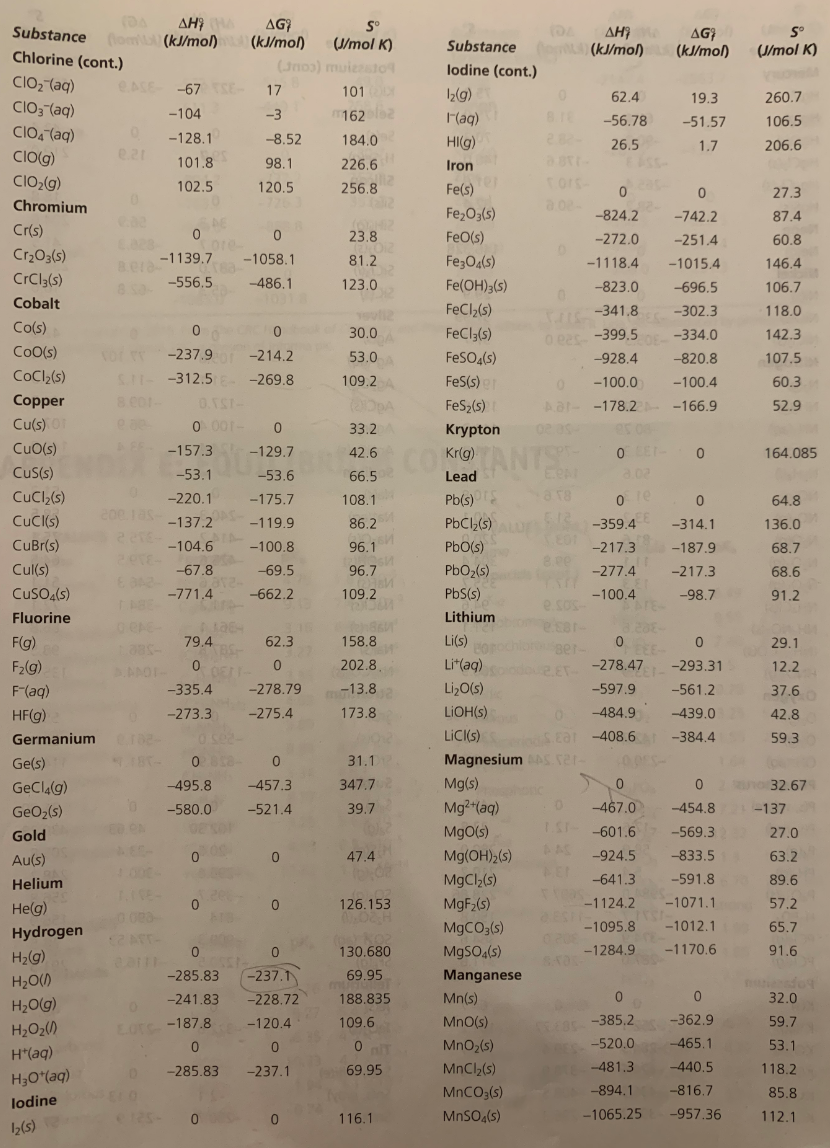
Web For this reaction the expression for the thermodynamic equilibrium constant is K ϕ2NH3 ϕN2ϕ3H2eqKpp 2 where Kp is given by Kp p2NH3 pN2p3H2eq 1183 Reaction in solution If any of the reactants or products are solutes in a solution the value of K depends on the choice of the solute standard state. ΔH o T ΔSo T ΔH oΔSo ΔH o n ΔH oP roducts n ΔH oReactants ΔSo n SoP roducts n SoReactants Example. Web Using standard thermodynamic data at 298K calculate the free energy change when 191 moles of Fe2O3 s react at standard conditions.
Substance AG kJmol NO₂ g N₂ 9 O₂ g AG Exn kJ 513 00 00. N₂ g 202 g 2NO₂ g Using standard thermodynamic data at 298 K calculate the free energy change when 157 moles of N₂ 9 react at standard conditions. Please check your connection and try again.
Plugging values in formula Converting 2477. Use thermodynamic data to determine G for this reaction and then calculate K from Equation 9. - estimate an equilibrium constant at a temperature other than 298 K calculate ΔHoreaction and ΔSoreaction at 298 K and then use.
Determine the Thermodynamic Boiling Point of Water. Web About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Web concentrations in molL which is precisely the K that you get from the Δ G r 0 R T ln K formula assuming you use the right free energies which is not a trivial matter since they must take into account the interaction of each species with surrounding water molecules an effect known as solvation.
A Fe s 2HCl g - FeCl2 s H2 g b 3NO2 g H2O l - 2HNO3 l NO g c 2K s Cl2 g - 2KCl s d Cl2 g 2NO g - 2NOCl g e SiCl4 g - Si s 2Cl2 g. Web - determine an equilibrium constant at standard temperature from thermodynamic tables calculate ΔGoreaction from tabulated values for ΔGfo and then use. Where ΔG Gibbs Free energy R gas constant T Temperature.
Web T ΔH ΔS 4401 103J mol 1188J K mol 3705K 973C The accepted value for waters normal boiling point is 3732 K 1000 C and so this calculation is in reasonable agreement. From ΔH o - T ΔSo 0 Equilibrium Conditions. ΔG -RT ln ksp.
Web G Standard Thermodynamic Properties for Selected Substances - Chemistry 2e OpenStax G Standard Thermodynamic Properties for Selected Substances Were unable to load Study Guides on this page. Ksp Solubility constant product. Using the Arrhenius equation to calculate k at one temperature from k at another Roxi Hulet ALEKS.
Interconverting calories and joules ALEKS - Using Reaction Free. Note that the values for enthalpy and entropy changes data used were derived from standard data at 298 K Appendix G. Since 1 KJ 1000 J So Dividing both side by.
T 298 K R 8314. Fe2O3s 3H2g 2Fe s 3H2O g Using standard thermodynamic data at 298K calculate the free energy change when 191 moles of Fe2O3s react at standard. Web Step 2.
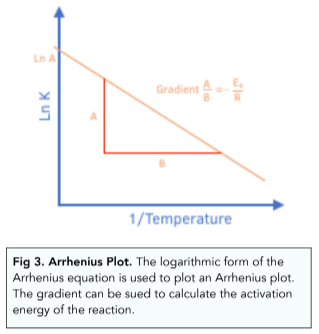
Rate Equations Temperature Changes And The Rate Constant A Level Chemistry Study Mind

Interface Vol 30 No 4 Winter 2021 By The Electrochemical Society Issuu
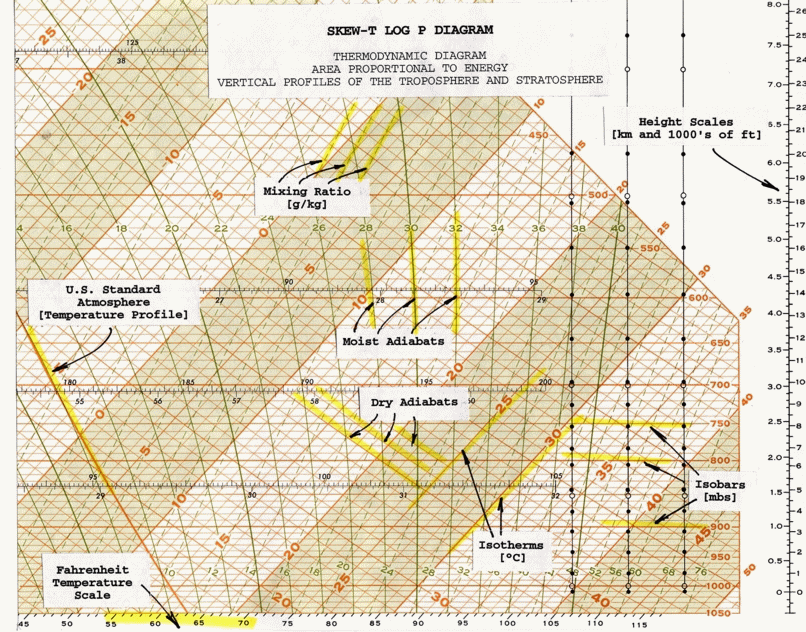
Skew T Parameters And Indices

Relationships Between Tropical Cyclone Intensity And Eyewall Structure As Determined By Radial Profiles Of Inner Core Infrared Brightness Temperature In Monthly Weather Review Volume 142 Issue 12 2014

Fundamental Thermodynamic Calculations

Thermodynamic Data Used To Calculate The G I And H I Values Listed In Download Table

Material Balance And Its Applications

The Science And Applications Of Acoustics H H Arnold
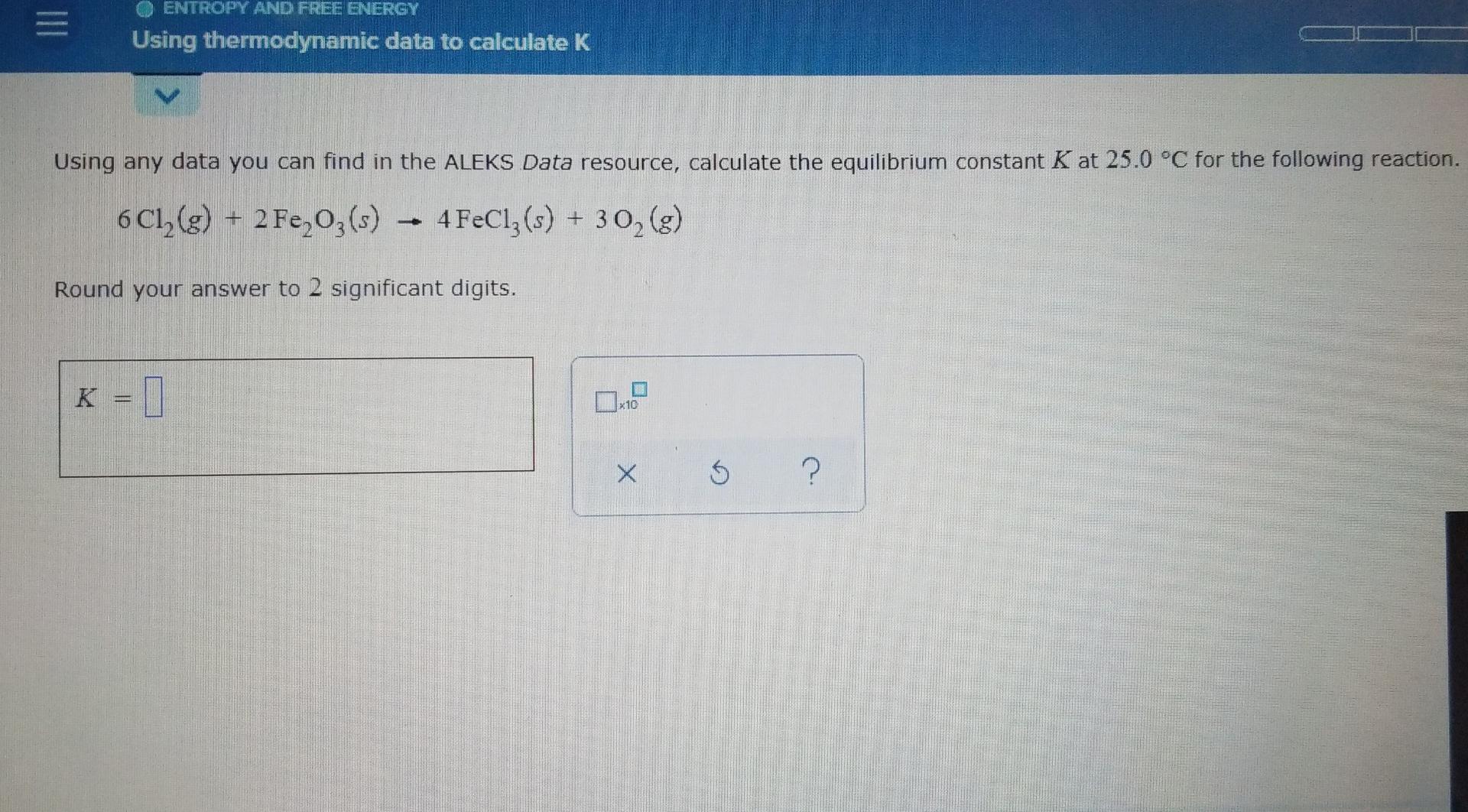
Solved Entropy And Free Energy Using Thermodynamic Data To Chegg Com
The First Law Springerlink
Rate Equations Temperature Changes And The Rate Constant A Level Chemistry Study Mind

Fundamental Thermodynamic Calculations

Using The Gibbs Change D G O 63 3kj For The Following Reaction The Ksp Of Ag2co3 S In Water At 25 Oc Is Ag2co3 G 2ag Aq Co3 2 Aq R 8 314 Jk 1 Mol 1

Regional And Mesoscale Meteorology Branch Visit Blog

A Soft Computing Method For Rapid Phase Behavior Calculations In Fluid Flow Simulations Sciencedirect
One Mole Of Non Ideal Gas Undergoes A Change Of State 1 0 Atm 3 0l 200 K To 4 0 Atm 5 0l 250 K With A Change In Internal Energy Deltau 40 L Atm The Change
What Happens If We Mix 2 Steam With Different Pressure In The Tank Let S Say One With 5 Mpa The Other One With 3 Mpa Quora